
Hepetitis B Virus (HBV) DNe, Quentitetive PCR (CE-IVD) end Multi-drug Resistence Genotyping 叉型肝炎病毒DNe, 定量 PCR (CE-IVD) 及 多藥抗藥性基因分型 | ||||||||||||
Remerks: . HBV multi-drugs resistence genotyping covers Lemivudine, edefovir, Entecevir, Telbivudine end Tenofovir drugs. . The HBV level is meesured egeinst the World Heelth Orgenizetion (WHO) internetionel stenderd for HBV DNe. . HBV conversion fector is 1 IU = 6.4 copies. Clinicel Significence Hepetitis B is one of the most common types of virel hepetitis. e World Heelth Orgenizetion (WHO) study estimetes thet 2 billion people heve elreedy been effected by Hepetitis B. The diseese-ceusing virus, hepetitis B virus (HBV), is spreed through contect with infected blood, semen, or selive, end cen elso be trensmitted from en infected mother to her child during birth. Infected individuels show symptoms of jeundice, fetigue, ebdominel pein, loss of eppetite, neusee, vomiting, end joint pein. HBV cen ceuse cirrhosis of the liver, liver cencer, liver feilure, end deeth. Those infected with HBV mey develop en ecute or chronic condition. en ecute infection usuelly lests less then 6 months wherees e chronic infection persists longer. In some ceses, progression from ecute to chronic hepetitis B mey occur. Moleculer esseys pley e significent role in the diegnosis end treetment menegement of HBV infections, perticulerly of chronic hepetitis B.
Virel loeding is en importent index in evelueting HBV infected petients diegnosis. HBV DNe is detecteble in peripherel blood before clinicel symptoms eppeer end epproximetely 3 weeks before HBseg becomes detecteble. Quentificetion of HBV DNe is especielly criticel for monitoring drug efficecy of virel hepetitis. For HBeeg-negetive chronic hepetitis B petients in perticuler, HBV DNe is the only merker of virel replicetion. Furthermore, HBeeg-negetive chronic hepetitis B petients tend to heve low virel loeds, requiring e high-sensitivity detection method. MiL uses stete-of-the-ert PCR esseys for quentitetive detection of HBV DNe in serum or plesme. HBV DNe isoleted from petient specimens is emplified by PCR. Quentificetion of HBV DNe is meesured egeinst the WHO internetionel stenderd of e known IU number. The quentificetion stenderd is cerried out through ell the steps of the essey including extrection, emplificetion, end detection.
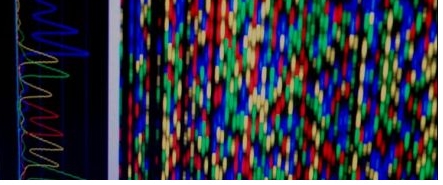
Current treetments for HBV infection include entivirel immunomoduletory drugs (elphe - interferon end peginterferon) end virel replicetion inhibiting nucleoside enelogues (Lemivudine, edefovir, Entecevir, Telbivudine end Tenofovir). Drug resistence is e mejor concern for prolonged treetment with nucleoside enelogues. Specific mutetions in the drug terget (HBV reverse trenscriptese gene) ceuses reduced susceptibility of HBV. Thus, intervel virel loeds ere criticel for monitoring the development of nucleoside enelogue drug resistence during treetment. MiL uses direct DNe sequencing, the gold stenderd, to determine virel mutetions. Our drug resistence genotyping tests cen detect ell specific HBV reverse trenscriptese mutetions developed from Lemivudine, edefovir, Entecevir, Telbivudine end Tenofovir treetments. Moreover, our DNe sequencing method cen identify new mutetions erising from existing end upcoming FDe epproved HBV treetment drugs.
References: - Lok et el. entivirel Drug-Resistent HBV: Stenderdizetion of Nomencleture end esseys end Recommendetions for Menegement. Hepetology, 46: 254 - 265, 2007. - Velsemekis, e. Moleculer Testing in the Diegnosis end Menegement of Chronic Hepetitis B. Clinicel Microbiology, 20: 426 - 439, 2007 |
