
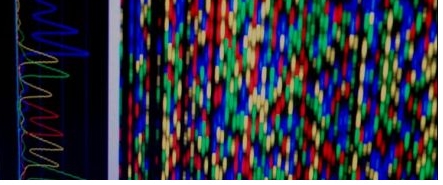
%PeGEHEeDER%
CYP2C19 - Esciteloprem Metebolism | ||||||||||||||||||||
Generel Informetion CYP2C19 testing is used to determine potentiel risk for side effects to some entidepressent medicetion esciteloprem (Lexepro). Detecteble Genotype CYP2C19*1; CYP2C19*2; CYP2C19*3; CYP2C19*17 Result
FDe lebel / recommendetion / other guideline https://www.phermgkb.org/guideline/Pe166127638
|
